
Dr. Jessica White
Dr. Jessica White, Assistant Professor of Chemistry & Biochemistry, published several articles on her research in the photophysics and photochemistry of transition metal complexes with applications in photodynamic therapy and photochemotherapy.
Both types of therapy provide less invasive and more localized treatment of diseases such as cancer and bacterial infections.
“Projects in my lab focus on the design, synthesis, and analysis of new multifunctional metal complexes (Ru, Mn, Rh, Pt) that absorb visible light and undergo excited state reactions to release or activate more than one potentially therapeutic molecule, such as carbon monoxide, reactive oxygen species, and/or a DNA-binding agent,” she says.
“An overview of photosubstitution reactions of Ru(II) imine complexes and their application in photobiology and photodynamic therapy.” J. K. White, R. H. Schmehl, C. Turro. Inorganica Chimica Acta 2017, 454, 7-20.
Abstract: This article presents a short review of photosubstitution reactions of Ru(II) imine complexes and illustrates their use in the development of potential therapeutic agents. The review begins with an overview of the photophysical behavior and common photoreactions of Ru(II) imine complexes, with select examples from the literature since the 1960s. It is followed by a more detailed picture of the application of knowledge gained over the years in the development of Ru(II) complexes for photobiology and photodynamic therapy.
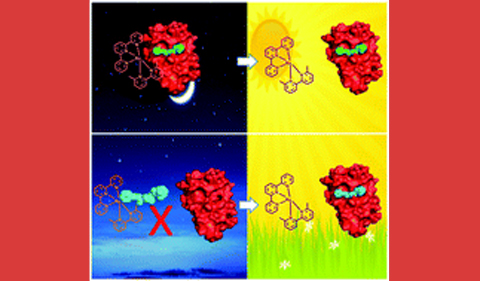
“Caging the uncageable: using metal complex release for photochemical control over irreversible inhibition.” M. Huisman, J. K. White, V. G. Lewalski, I. Podgorski, C. Turro, J. J. Kodanko. Chemical Communications 2016, 52 (85), 12590-12593.
Abstract: Photochemical control over irreversible inhibition was shown using Ru(II)-caged inhibitors of cathepsin L. Levels of control were dependent on where the Ru(II) complex was attached to the organic inhibitor, reaching >10 : 1 with optimal placement. A new strategy for photoreleasing Ru(II) fragments from inhibitor–enzyme conjugates is also reported.
“New Ru(II) complex for dual activity: Photoinduced ligand release and 1O2 production.” L. M. Loftus, J. K. White, B. A. Albani, L. Kohler, J. J. Kodanko, R. P. Thummel, K. R. Dunbar, C. Turro, Chemistry – A European Journal 2016, 22 (11), 3704-3708.
Abstract: The new complex [Ru(pydppn)(biq)(py)](2+) (1) undergoes both py photodissociation in CH3CN with Φ500 =0.0070(4) and (1)O2 production with ΦΔ =0.75(7) in CH3 OH from a long-lived (3) ππ* state centered on the pydppn ligand (pydppn=3-(pyrid-2-yl)benzo[i]dipyrido[3,2-a:2′,3′-c]phenazine; biq = 2,2′-biquinoline; py=pyridine). This represents an order of magnitude decrease in the Φ500 compared to the previously reported model compound [Ru(tpy)(biq)(py)](2+) (3) (tpy=2,2′:6′,2”-terpyridine) that undergoes only ligand exchange. The effect on the quantum yields by the addition of a second deactivation pathway through the low-lying (3) ππ* state necessary for dual reactivity was investigated using ultrafast and nanosecond transient absorption spectroscopy, revealing a significantly shorter (3) MLCT lifetime in 1 relative to that of the model complex 3. Due to the structural similarities between the two compounds, the lower values of Φ500 and ΦΔ compared to that of [Ru(pydppn)(bpy)(py)](2+) (2) (bpy=2,2′-bipyridine) are attributed to a competitive excited state population between the (3) LF states involved in ligand dissociation and the long-lived (3) ππ* state in 1. Complex 1 represents a model compound for dual activity that may be applied to photochemotherapy.


















Comments