
Dr. Eric Masson
Dr. Eric Masson, Associate Professor of Chemistry & Biochemistry, was awarded a two-year grant for $110,000 from the American Chemical Society Petroleum Research Fund in March 2016 to study the encapsulation of hydrocarbons into Cucurbiturils, a family of hollow pumpkin-shaped molecules that the Masson group has been investigating since 2008.
Optical methods are commonly used to identify the source of crude oil batches and to predict some of their physicochemical properties. However, improving the scope and quality of the prediction remains challenging, as it necessitates more sensitive and composition-specific assays. In this work, the Masson group lays the fundamental groundwork of a long-term goal: the development of hydrocarbon-specific supramolecular assays with enhanced characterization and prediction abilities.
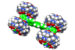
The objective in this study is to identify the binding forces between various hydrocarbons and a series of auxiliary organic units used as probes with tunable properties. The Cucurbit[8]uril macrocycle (CB[8]) is used to bring together the hydrocarbon and an auxiliary unit in its cavity. The auxiliary unit is connected to a fluorinated metal-ligand complex that generates an optical response, and is used to quantify hydrocarbon binding by 19F nuclear magnetic resonance spectroscopy.
![Cucurbit[8]uril macrocycle](https://www.ohio-forum.com/wp-content/uploads/2016/11/Cucurbit8uril-macrocycle.jpg)
Ramin Rabbani, a second-year graduate student in the Masson group, recently obtained extremely encouraging results, and showed that cyclopentane and cyclohexane (see Figure above, molecule in yellow) could be encapsulated into CB[8] together with a tolyl probe (in violet) anchored to a fluorinated ruthenium complex. Rabbani is now testing the selectivity of his probe toward a wide range of hydrocarbons, and is looking forward to start writing his first publication in the coming weeks.


















Comments